Our Research
For the most up-to-date examples of our research, check out our Publications page.
For a sample of some of our many projects, keep reading!
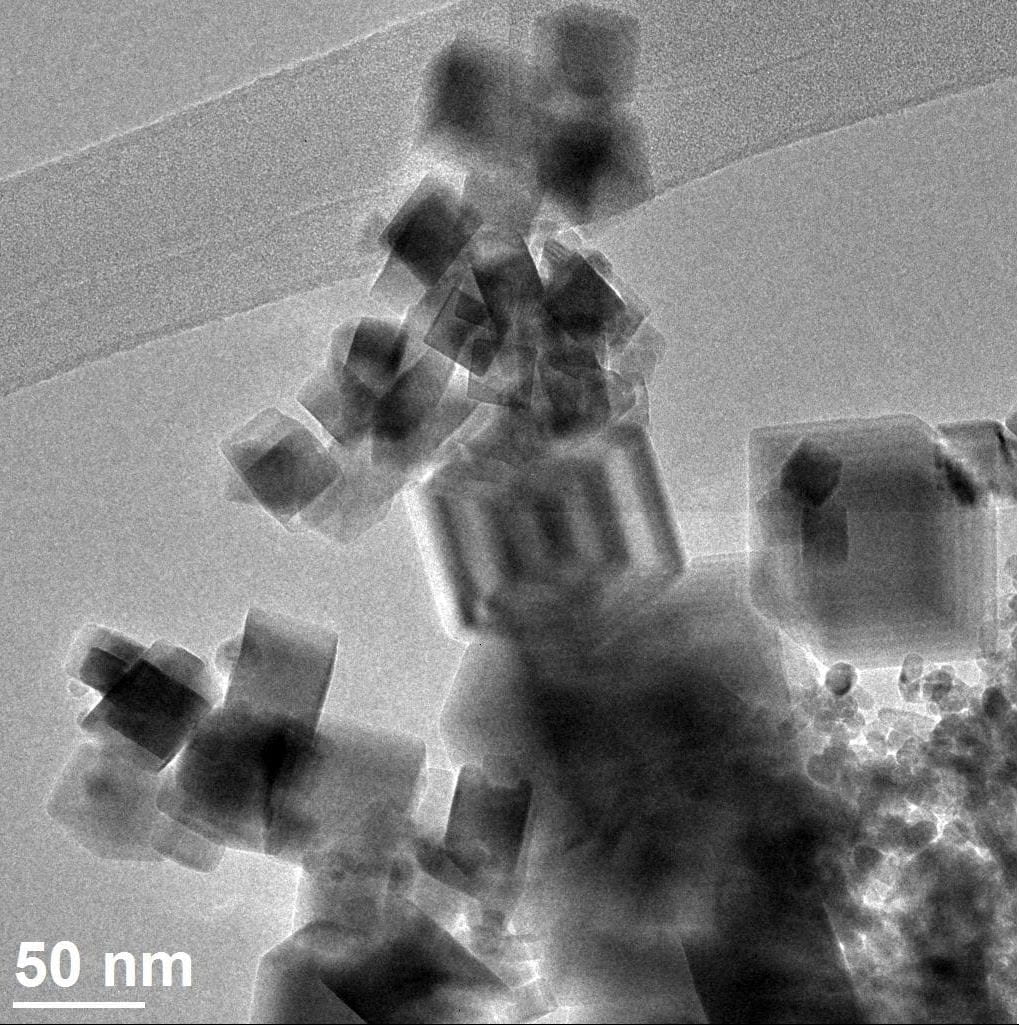
Precision Oxide Supports and Catalysts
-
Bulk oxides can be used as catalysts and supports, and their structures play an important role in dictating reactivity.
-
We have extensive work on ceria-based materials, including different nanoshapes, clusters, and 2D materials.
-
We have also worked to understand how additional active sites (VOx, FeOx, CoOx) are grafted onto these redox-active solids.
-
Through collaboration, we have examine novel lanthanide scandates and metal titanates as catalyst supports.
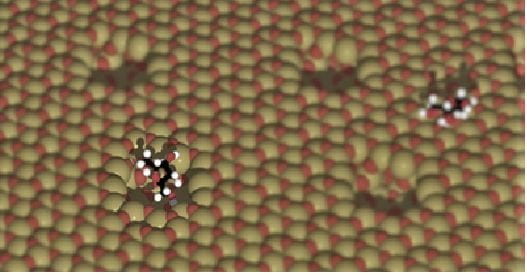
Overcoating Metal Oxides for Shape Selectivity and Metal Nanoparticle Stabilization
-
We are developing new shape-selective, all-oxide catalysts that we call nanobowls. We are learning to control size, shape, and even chirality.
-
These materials consist of < 2 nm cavities on the surface of an oxide carrier particle that exists as controlled voids in an overcoated layer. They may be of a variety of compositions.
-
The overcoating concept is being extended to control selectivity and improve stability of a number of catalyst classes including supported metal nanoparticles, acid catalysts, and tandem catalysts.
-
Variations include developing novel methods to entrench or to overcoat active sites in collaboration with other research groups.

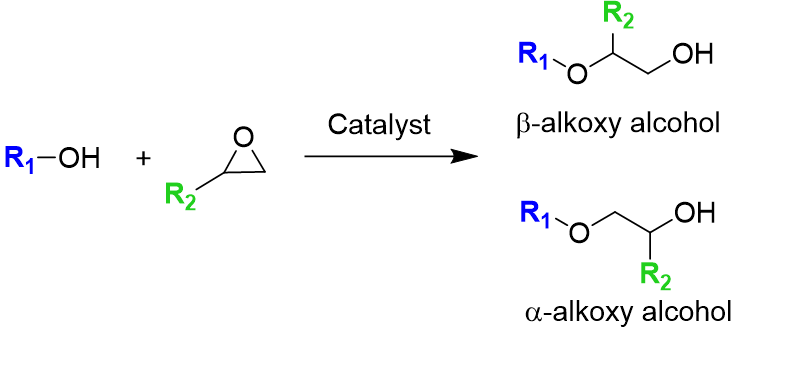
Selective Ring-Opening Catalysis with Molecular Lewis Acid Catalysts (with Dow)
-
Selective ring-opening of epoxides leads to a number of useful products or synthons and is a useful probe reaction for understanding acid catalysts.
-
We use computational techniques and experimental data to develop homogeneous and heterogeneous regioselective catalysts and to optimize reaction conditions.
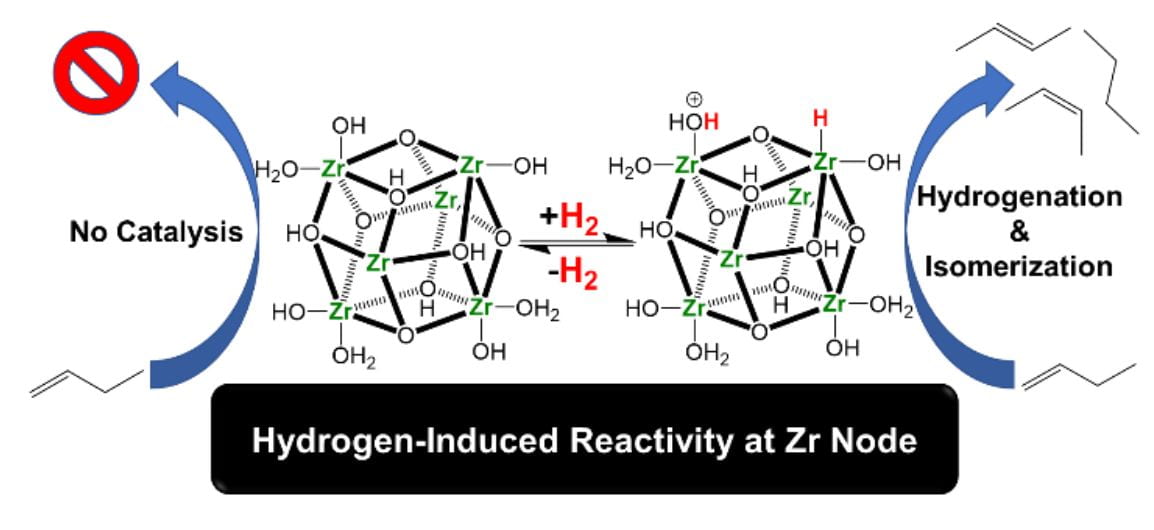
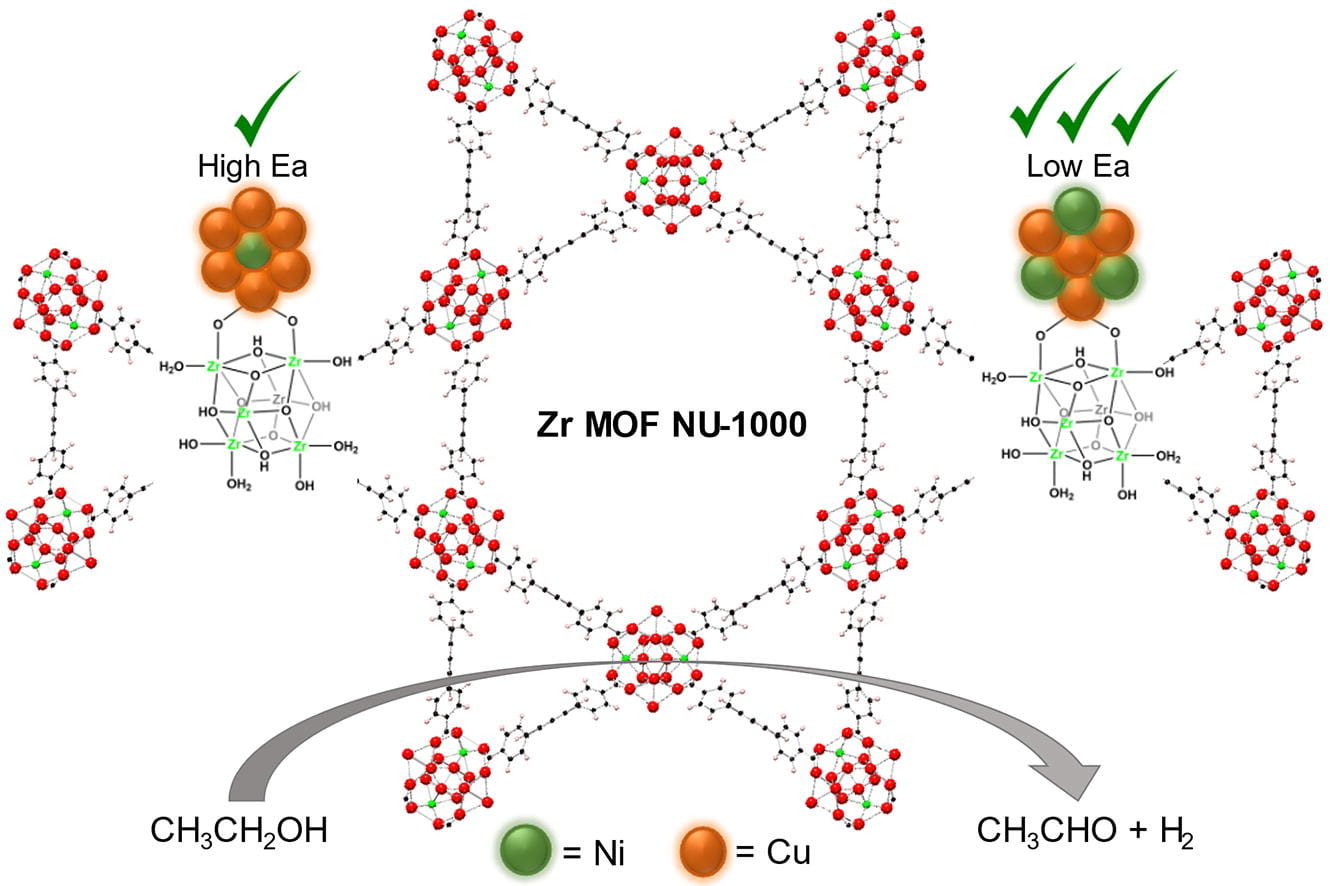
Metal-Organic Frameworks (MOFs) as Heterogeneous Catalysts and Supports
-
MOFs offer the potential for uniform distributions of isolated catalytic active sites that can be compared to conventional supported catalysts.
-
MOFs also offer a broader range of control over the local pore environment than do zeolites or conventional oxide supports. This can have an especially important role for condensed phase reactions.
-
Currently, we use probe reactions such as non-oxidative ethanol dehydrogenation, butene hydrogenation and isomerization to understand the nature of isolated catalytic active sites in MOF systems.
Well-defined metal sulfides (and oxides!) for dehydrogenation
-
We have shown them to be active for alkane dehydrogenation and alcohol dehydrogenation and acceptor-less dehydrogenative coupling
-
Metal sulfides are much less studied for dehydrogenation.
-
Metal sulfides are known hydrogen evolution catalysts and are used frequently in hydrogenation/hydrogenolysis (hydrotreating)
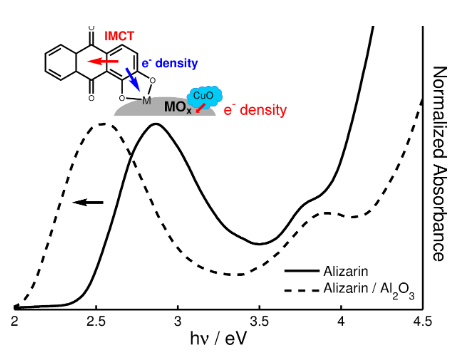
Supported copper oxide as a new catalyst for oxidative dehydrogenation
- We are also learning to understand support effects via Lewis acidity dye probes.
- At low loadings, it is an active material for oxidative dehydrogenation with significant support effects and differences from other supported oxides
- Supported copper oxide is often inactive or is prone to complete reduction to metal at high loadings

SiO2 deposition on metal oxides to tune solid acid behavior
-
Metal oxide catalysts including zeolites (SiO2-Al2O3) usually contain a mixture of sites and pore structures
-
Targeted catalyst design is in high demand for application in natural gas and biomass conversion processes
-
NRG has developed a liquid phase SiO2 overcoat technology and applied this to Al2O3 and Ti-SiO2 catalysts
Contact Us
Professor Justin Notestein
Department of Chemical & Biological Engineering
